Drug Selection
Immuno-Oncology is a rapidly growing area of tumor therapy that fights cancer by:
- Recruiting the patient’s immune system.
- With drugs that are based on immune mechanisms.
- Antibodies
- Cancer vaccines.
- Immune cells engineered to recognize cancer cells (for example, CAR T cells).
- Immune checkpoint inhibitors.
To order tests or clarify questions, please reach us via email info@plexision.com or call us at +1 (855) 753-9474.
Plexision’s functional assays characterize tumor antigenicity, anti-tumor responses, and their modulation with specific immune checkpoint inhibitors. These assays use tumor cells or purified tumor antigens and can be used in clinical trials.
- Upon antigen recognition by an immune cell, the IMMUNE RESPONSE to a foreign antigen is shaped by the balance between inflammatory and inhibitory co-stimulation (Fig. 1).
- The anti-tumor response requires that a tumor cell be recognized as foreign, or immunogenic (antigenic).
- The anti-tumor activity also requires T-cells to launch a cytotoxic immune response toward tumor cells. Prior chemotherapy may result in T-cell exhaustion and limit T-cell response.
- Tumor cells send predominantly inhibitory signals to T-cells. As a result, T-cells can no longer ‘see’ the tumor cell (Fig. 2).
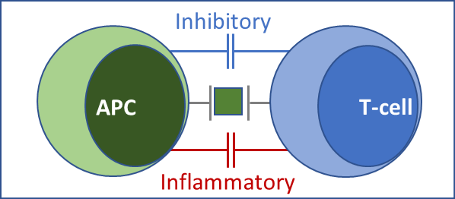
Fig 1. IMMUNE RESPONSE Antigen-presenting cells (APC) present antigen to T-cells. Simultaneously, inflammatory and inhibitory costimulatory signals are generated by respective receptor-ligand combinations, which serve as immune checkpoints. The final immune response, whether cytotoxic (inflammatory), or suppressive (inhibitory), represents a balance between the two.
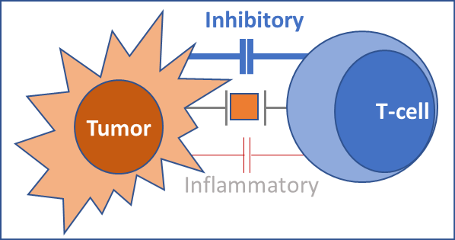
Fig 2. TUMOR DEFENSE AGAINST IMMUNE RESPONSE: Tumors overexpress inhibitory costimulatory proteins, such as PD-L1. Inhibitory signals are communicated to T-cells via complementary T-cell-bound receptors such as PD-1. As a result, inhibitory signaling to T-cells is enhanced. Inflammatory signals are overwhelmed. The tumor escapes an immune attack.
Tumor Immunotherapy with checkpoint inhibitors
A whole blood sample must be collected in a sodium heparin (green-top) tube.
- Immune checkpoint inhibitors suppress tumor-derived inhibitory signals, making tumor cells ‘visible’ to the immune system (Fig. 3).
- The patient’s immune system can, therefore ‘see’ the tumor, and mount an anti-tumor immune response to eliminate it.
- These drugs have dramatically improved the landscape of cancer therapy. However, efficacy failures have occurred during some clinical trials. Debilitating side effects have also occurred due to a non-specific immune response against normal tissues and organs.
Fig. 3. CHECKPOINT INHIBITORS & ANTI-TUMOR IMMUNE RESPONSE: Inhibitors such as anti-PD1 bind to and neutralize T-cell-bound inhibitory receptors such as PD-1. Others like anti-PD-L1 bind to and neutralize inhibitory ligands such as PD-L1, which are overexpressed on tumor cells. This binding suppresses inhibitory signals. As a result, inflammatory signals are enhanced. The tumor undergoes an immune attack and is eliminated. Autoimmune side effects are also possible.
Challenges and Unmet Needs
- To maximize benefit and minimize risk, it is critical to know whether a particular checkpoint inhibitor is effective in augmenting the anti-tumor immune response in a given patient.
- To determine whether a checkpoint inhibitor may be effective, immune staining is used to determine whether its target is expressed by the tumor. To determine whether a tumor is antigenic and may therefore elicit an immune response, tumor DNA sequencing is used to identify novel antigens or neoantigens, which are absent in normal tissues.
- These methods characterize the tumor phenotype but are not foolproof. Several checkpoints act simultaneously to modulate anti-tumor immune responses. For this reason, the dominant checkpoint by which a tumor escapes immune attack may not be targeted by a particular inhibitor. Further, whether the immune system ‘sees’ the tumor as antigenic, and whether the anti-tumor response is enhanced by a checkpoint inhibitor cannot be determined by phenotypic characterization.
Personalized Immunotherapy
- Functional assays that provide an integrated view of all possible checkpoints that are operative can complement current methods to select the right drug for each patient in clinical trials and clinical practice.
- Plexision’s functional assays characterize tumor antigenicity and anti-tumor responses and their modulation with specific immune checkpoint inhibitors. These assays use tumor cells or purified tumor antigens and can be used in clinical trials.
- Plexision’s functional test systems are performed in its GLP- and cGMP-compliant CLIA-approved reference laboratory.
