COVID-19 variant: Early data
A new variant of the coronavirus that causes COVID-19 has been reported in the UK. This variant was detected by the Covid-19 Genomics UK (COG-UK) consortium.
Source: https://www.bmj.com/content/371/bmj.m4857
A simplified overview of early data is available at the following link
Key Details:
- The variant is called B.1.1.7 or variant under investigation, the year 2020, month 12, variant 01 (VUI 202012/01)
- The rate of transmission is 71% higher than other variants
- The first case occurred on September 20, 2020
- Sequenced in early October 2020
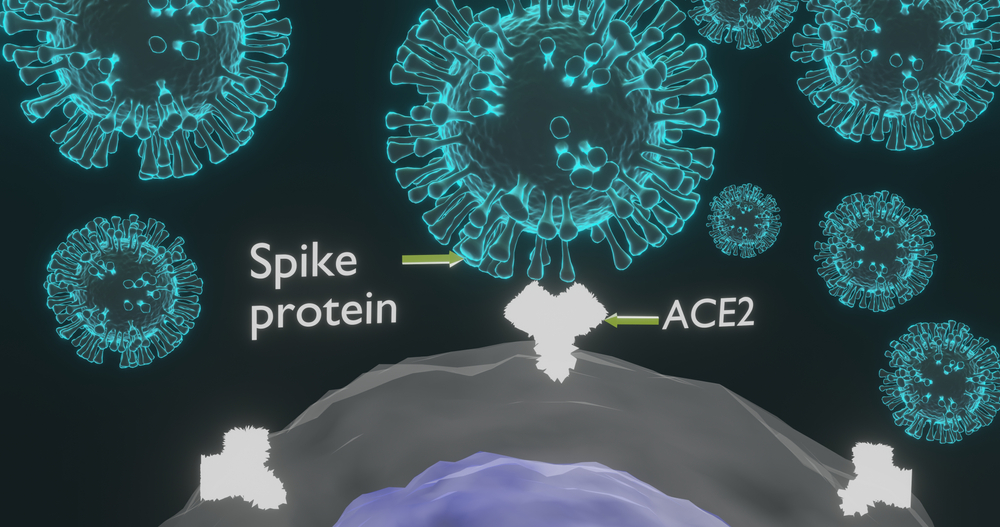
- The variant has 17 new mutations, 8 in the spike protein which binds to human cells. Mutations have caused amino acid changes and deletions
- Mutation N201Y in the receptor-binding domain of the spike protein renders the virus more avid for its human receptor, ACE2.
- The deletion at positions 69/70 affects the performance of some diagnostic PCR assays
- Current vaccines should be effective against this variant
- The variant does not affect the severity of COVID-19
- Children are more likely to be affected, because the variant is more contagious, and schools have remained open in some areas
- Several countries have begun to see cases with this variant
Source: https://www.bmj.com/content/371/bmj.m4944
Immunocompromized patients
- Some mutations seen in the variant may have occurred in immunocompromised patients
Source: https://www.medrxiv.org/content/10.1101/2020.12.05.20241927v2.supplementary-material
Mathematical modeling by a group of researchers predicts a higher transmission rate than pre-existing variants of SARS-CoV-2, without clear evidence of altered disease severity. These models project increased spread unless vaccination roll-outs are accelerated, and school-based transmission is reduced.
Source: https://cmmid.github.io/topics/covid19/uk-novel-variant.html