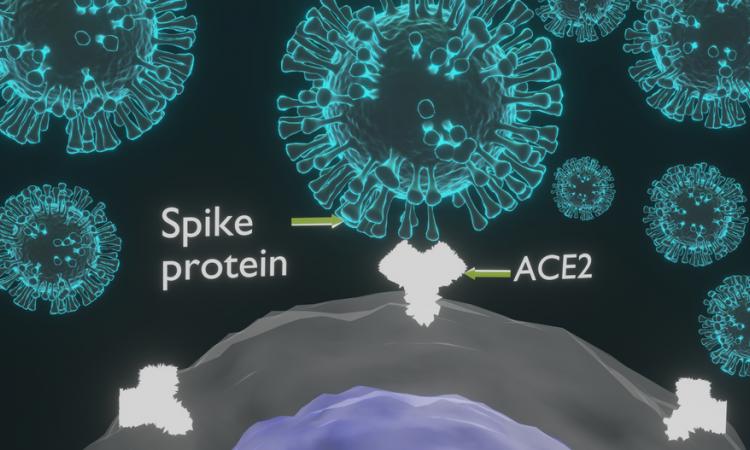
An exploratory study reported on May 23, 2021, in MedRxiv described changes in T-cell and antibody immunity after mRN
About
Plexision develops cellular biomarkers for personalized diagnosis and drug development in solid organ transplantation and immunological disorders. We also pioneer in R&D projects centered on integrating biomarker targets in all phases of drug development, from preclinical to post-marketing. Plexision’s technology can be adapted to
- - Assess disease risk for several immunological disorders.
- - Predict the success of a drug for a specific patient.
- - Develop dosing recommendations for new immunological drugs.
Our state-of-the-art laboratory is CLIA-certified and located in Pittsburgh, PA.
Contact
- +1 (855) 753-9474
- info@plexision.com
- 4424 Penn Ave., #202, Pittsburgh, PA 15224 USA