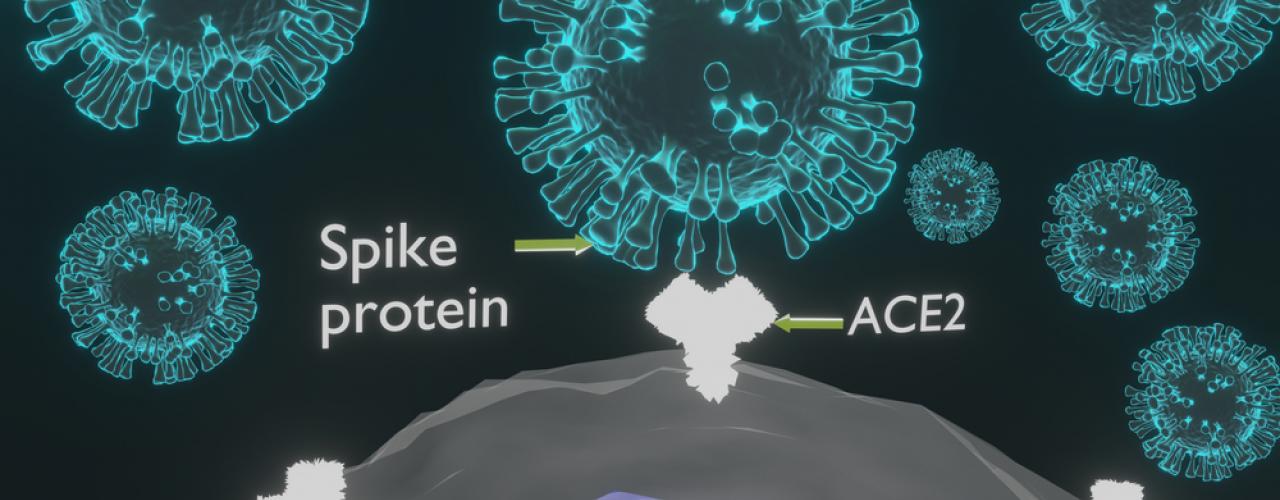
- On February 27, the FDA issued an Emergency Use Authorization for John
About
Plexision develops cellular biomarkers for personalized diagnosis and drug development in solid organ transplantation and immunological disorders. We also pioneer in R&D projects centered on integrating biomarker targets in all phases of drug development, from preclinical to post-marketing. Plexision’s technology can be adapted to
- - Assess disease risk for several immunological disorders.
- - Predict the success of a drug for a specific patient.
- - Develop dosing recommendations for new immunological drugs.
Our state-of-the-art laboratory is CLIA-certified and located in Pittsburgh, PA.
Contact
- +1 (855) 753-9474
- info@plexision.com
- 4424 Penn Ave., #202, Pittsburgh, PA 15224 USA